~~TOC~~
How to Perform Antibunching Measurements
Antibunching Measurements are (normally) measured in T2 mode. In T2 mode, all input channels of the TCSPC electronics are treated the same, also the “Sync” channel. Therefore the sync has to be disabled either by disconneting the cable, or by changing the CFD level or trigger level to such a value that the sync is not recognized any more. i.e. Sync rate = 0 . Directly in the Sepia software, the sync out can also be disabled.
The descriptions below only mention physically disconnecting the sync. This is strictly necessary only for the PicoHarp 300 because it only has two channels.
Emission Filters
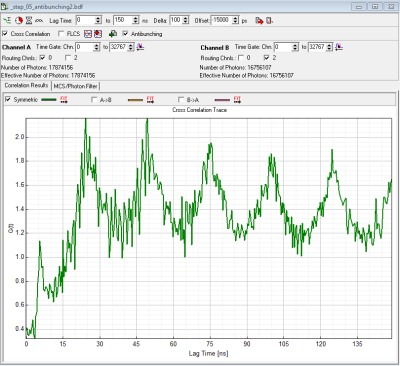
During antibunching measurements, the avalanche breakdown flash of SPADs may become visible as unexpected additional peaks near the expected antibunching dip (see figure 2). These peaks might be useful to find the location of the dip, they are however artifacts caused by photons emitted (after the detection of a genuine photon) by one of the SPADs, detected by the second SPAD. The distance of the expected dip and the afterflash peak is characteristic for the setup.
To get rid of the afterflash peak, it has been proven useful to introduce 2 identical bandpass filters directly in front of each SPAD E.g. the afterflash of MPD SPADs for Atto488 (485 nm excitation, emission around 520 nm) could be suppressed by inserting one 536/40 bandpass filters in front of SPADs.
We also recommend to use a 50:50 beam splitter plate instead of a beam splitter cube, or to intentionally misalign the detectors slightly.
Antibunching using SymPhoTime
- with PicoHarp 300 in T3-mode
- not possible with SymPhoTime (32-bit version), because in SPT32 the Sync Divider is always active and set to 8
Instead, data acquisition is possible with native PicoHarp 300/HydraHarp 400 softwares as well as with SymPhoTime64, where the sync divider can be disabled.
Using a PicoHarp300, connect one detector to Ch.0 (SYNC) and the other one to Ch.1
With a HydraHarp400, don't use use SYNC channel as input for one detector. Use e.g. input channels 1 and 2. The SYNC should stay connected.
Unfortunately, analysis of HydraHarp400 T3-mode antibunching data is not implemented in SPT.
-
- measurements are possible with SymPhoTime64
Changing from Normal Acquisition (T3-mode) to Antibunching (T2-mode)
Cabling: PicoHarp 300
- Disconnect Sync from PicoHarp 300 Ch.0.
- Disconnect SPAD 1 Signal from PHR800 Router.
- Connect SPAD 1 Signal to PicoHarp 300 Ch.0 only if it is NIM compatible. If the SPAD provides only TTL output pulses, you need to use the SIA400 adapter.
- Adjustment of the CFD Level for Ch 0 (Sync Channel) might be necessary.
It is also possible to connect both detectors directly to the PicoHarp 300 without passing SPAD 2 through the router. In this case, however, the router cable (DVI-cable) needs to be disconnected from PicoHarp300
Cabling: HydraHarp 400
- Disconnect the Sync signal from HydraHarp 400 SYNC IN. (That is all.)
- if the laser is an LDH-series heasd driven by SEPIA II driver, it's enough to disable the SYNC in the SEPIA software ⇒ no cable change necessary.
Software Settings during Acquisition
- diffusing Samples: typically just point measurement in T2-mode (see below)
-
- for example see Diamond NV centers
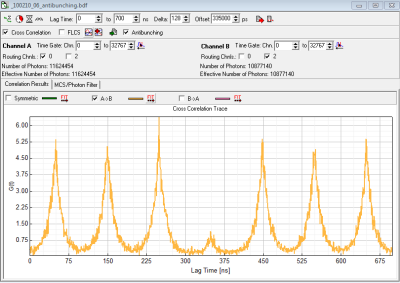
- depending on preference set laser repetition rate in order to allow full fluorescence to fully decay or highest possible rate or cw-excitation. The advantage of the highest possible repetition rate (or even cw) is better resolvability of the dip, disadvantage is that fluorescence does not decay completely during the pulses. (Compare figure 1 (10MHz) rep. rate and figure 2 (40 MHz) rep rate).
- Select T2-mode
- Start Point Measurement
- For a single immobilized molecule antibunching trace you will need at least a trace of 30s fluorescence emission with a minimum mean countrate of 10 kcps.
Analysis in SymPhoTime
- Select FCS Analysis
- Select CrossCorrelation and Antibunching
- Left Correlation Channel:
- PicoHarp 300: Select Ch0 (this is now SPAD 1)
- HydraHarp 400: Select Ch1 SPAD 1)
- Right Correlation Channel:
- PicoHarp 300 (SPAD 2 connected to Ch2 of the Router) Select Ch2 (SPAD 2)
- PicoHarp 300 (w/o Router) Select Ch1 (SPAD2)
- HydraHarp 400 select Ch2 (SPAD 2)
- LagTime To: select few multiples of the Laser Repetition Rate (e.g. for 10 MHz select 700 ns – so you will see 7 peaks)
- Delta is the step width of the lag-time axis in units of the PicoHarp 300 Resolution.
- e.g. for 32 ps Resolution Setting, selecting a Delta = 128 will result in approx. 4 ns steps for the x-Axis of the correlation.
- Start with lag-time/200/T2-resolution (e.g. 700ns/200/32ps ~=110; this means your x-scale will have 198 points, resolution will be 3.52 ns).
- Higher Delta values will speed up the calculation but result in a coarser correlation curve.
- Offset is the delay (in ps) between the two Detectors
- HydraHarp 400: in a MicroTime200 with HydraHarp detection both detectors including cables+ electronics have the same delay. Offset = 0 will show the dip at lagtime =0 (see figure 2).
- PicoHarp 300 in a MT200 with PH both detectors including cables+electronics have a different delay - in our case it is in the region of -15000 (15 ns) due to the router.
- However to move the expected “dip” to the center of the x-axis we here use the half of the x-axis range minus the difference in cable length (700ns/2 - 15 ns =335000 ps). (see figure 1)
- Start Correlation Calculation
The symmetric correlation, which is shown automatically will only show a small “dip” as it is the average of AxB AND BxA in the case of figure 1 AxB will have its dip at 350 ns and BxA will have its dip at -350 ns. Therefore only one of both correlation (here AxB) should be displayed.
Some MT200 based literature about Antibunching
- Yuan C. T., Yu P., Ko H. C., Huang J., Tang J.: Antibunching single-photon emission and blinking suppression of CdSe/ZnS quantum dots. In: ACS Nano. Vol. 03, p.3051-3056 (2009).
- Koberling F., Kraemer B., Buschmann V., Rüttinger S., Kapusta P., Patting M., Wahl M., Erdmann R: Recent advances in photon coincidence measurements for photon antibunching and full correlation analysis. In: Proceedings of SPIE. Vol.7185, 71850Q (2009).
- Fore S., Laurence T.A., Hollars C. W., Huser T.: Counting constituents in molecular complexes by fluorescence photon antibunching IEEE Journal of Selected Topics. In: Quantum Electronics. Vol.13, p.0996-1005 (2007)
- Nettels D., Gopich I. V., Hoffmann A., Schuler B: Ultrafast dynamics of protein collapse from single-molecule photon statistics. In: Proceedings of the National Academy of Sciences of USA. Vol.104, p.02655-02660 (2007)
- Ta H., Kiel A., Wahl M., Herten D.-P.: Experimental approach to extend the range for counting fluorescent molecules based on photon-antibunching Physical Chemistry Chemical Physics, Vol.12, p.10295-10300 (2010)